Enzyme
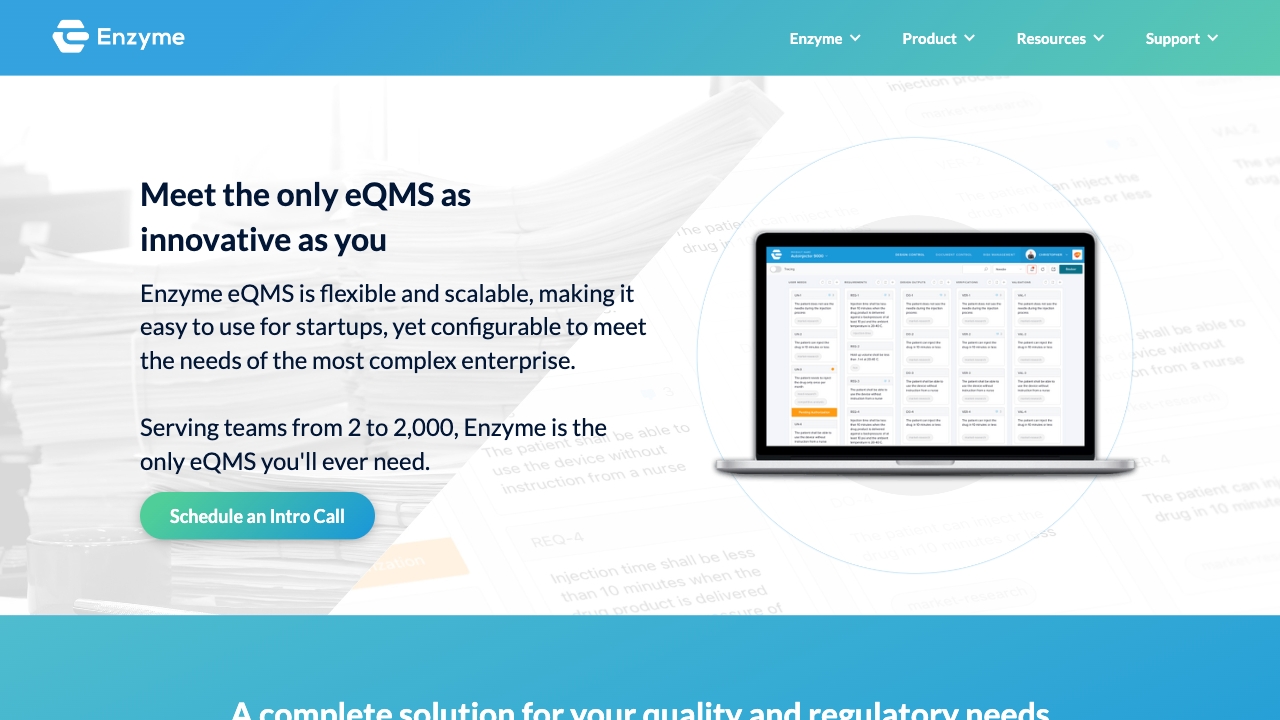
Enzyme is a QMS (Quality Management System) software that helps medical device, digital health, and biopharma companies streamline their quality processes and ensure compliance with industry standards such as cGMP, QSR, and ISO. It covers all stages of the product development lifecycle and offers a range of features to support document control, change control, training, risk management, audits, complaints, nonconformance, CAPA (Corrective and Preventive Actions), and more.
Description
how to use:
To use Enzyme, start by scheduling a demo to explore the software’s capabilities. Once onboarded, you can import your existing data and integrate Enzyme with your preferred tools for seamless data transfer. Use the core features like document control, change control, and training to manage your quality processes effectively. You can also utilize Enzyme’s design control, risk management, suppliers, audits, complaints, nonconformance, and CAPA features as per your organization’s specific needs. The system is designed to be intuitive and adaptable, allowing you to work in a way that suits your workflows.
Core freatures:
Document ControlChange ControlTrainingDesign ControlRisk ManagementSuppliersAuditsComplaintsNonconformanceCAPA
Use case:
Streamlining quality processes
Ensuring compliance with industry standards
Managing the product development lifecycle
Improving document control and change management
Enhancing training and risk management
Facilitating supplier management and audits
Effectively handling complaints and nonconformance incidents
Implementing corrective and preventive actions
FAQ list:


Reviews
There are no reviews yet.